Similar Compounds
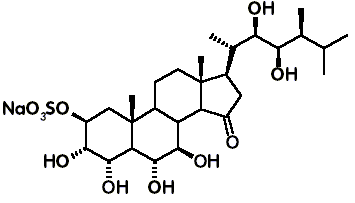
The tamosterones were isolated from
an Oceanapiid sponge of a new genus collected in the Tamil Channel (X. Fu,
M. L. G. Ferreira, F. J. Schmitz & M. Kelly, Tamosterone sulfates: a
C-14 epimeric pair of polyhydroxylated sterols from a new Oceanapiid sponge
genus. Journal of Organic Chemistry 64: 6706-6709, 1999). They
are similar to brassinosteroids as they are 3-oxygenated steroidal 22a,23a-diols, but are distinct from them
by presenting an oxygenation pattern of rings A, B and D not seen in natural
brassinosteroids, as well as 14b stereochemistry in 14-epitamosterone sulfate.
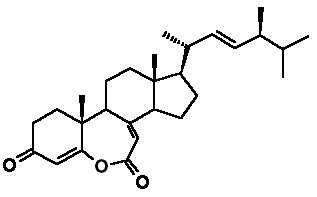
Asterasterol A (S. De Marino, E. Palagiano, F. Zollo, L. Minale
& M. Iorizzi, A novel sulfated steroid with a 7-membered 5-oxalactone
B-ring from an Antarctic starfish of the family Asteriidae, Tetrahedron
53: 8625-8628, 1997) and herbarulide (K. Krohn, C. Biele, H.-J. Aust,
S. Draeger & B. Schulz, Herbarulide, a ketodivinyllactone steroid with
an unprecedented homo-6-oxaergostane skeleton from the endophytic fungus
Pleospora herbarum, Journal of Natural Products
62: 629-630, 1999) are the two known natural steroids, besides brassinosteroids,
that present a lactone function in ring B, but with opposite regiochemistry
of that found in brassinosteroids.